Shire v Watson Pharmaceuticals
SHIRE DEVELOPMENT, LLC, SHIRE PHARMACEUTICAL DEVELOPMENT, INC., COSMO TECHNOLOGIES LIMITED, GIULIANI INTERNATIONAL LIMITED, NKA NOGRA PHARMA LIMITED,
Plaintiffs-Appellees v. WATSON PHARMACEUTICALS, INC., NKA ACTAVIS, INC., WATSON LABORATORIES, INC. – FLORIDA, NKA ACTAVIS LABORATORIES FL, INC., WATSON PHARMA, INC., NKA ACTAVIS PHARMA, INC., WATSON LABORATORIES, INC., Defendants-Appellants
2016-1785 February 10, 2017
Plaintiffs Shire sued Defendants Watson for infringing claims 1 and 3 of U.S. Patent No. 6,773,720. The Federal Circuit analyzed the Markush group requirements in claim 1(b). The Court strictly construed the claims as being “closed” due to the presence of the Markush group, reversing the district court with instructions to enter judgment of non-infringement.
The ’720 patent is directed to a controlled-release oral pharmaceutical composition of mesalamine. That composition includes the mesalamine active ingredient; an inner lipophilic matrix; an outer hydrophilic matrix; and other optional excipients. The inner lipophilic matrix and the outer hydrophilic matrix are expressed in the claim as Markush group.
This case has been to the Federal Circuit twice before: in 2014 (746 F.3d 1326), and 2015 (787 F.3d at 1366, remanded by 135 S. Ct. 1174 (2015)). Referencing the 2015 decision, the Court “explained that the matrix compositions are ‘limited by the Markush groups’ added during prosecution ‘to overcome the examiner’s rejection of the claims as obvious.’”
The district court concluded that Watson’s ANDA Product satisfied the “inner lipophilic matrix” and “outer hydrophilic matrix” limitations because the excipients fall outside the respective Markush groups. The district court found that the excipients were “unrelated” to the invention since they did not drive the water-affinity property of their respective matrices.
The Court emphasized that the presence of “consists of” or “consisting of” in a claim “creates a very strong presumption that that claim element is ‘closed’ and therefore exclude[s] any elements, steps, or ingredients not specified in the claim.’” The Court distinguished its holding in Norian Corp. v. Stryker Corp., 363 F.3d 1321, 1331 (Fed Cir. 2004), where the presence of a spatula in a calcium phosphate chemical kit did not take the accused product outside the scope of the asserted patent ”since (the spatula) has no interaction with the chemicals, and is irrelevant to the invention.”
The district court found that Watson infringed because magnesium stearate, a component outside of the Markush group, is unrelated to the invention. The Federal Circuit disagreed, concluding that the magnesium stearate retains its lipophilic character, that it “structurally and functionally relates to the invention, and [that] its presence in the outer matrix violates the ‘consisting of’ requirement in claim 1(b).”
The Court noted that “Norian did not restrict ‘related’ components to only those that advance or are intended to advance a Markush group’s allegedly inventive elements.” In the Court’s view, such a requirement “which would in effect equate the scope of a Markush group’s ‘consisting of’ language with either ‘comprising’ or ‘consisting essentially of’ language.”
Shire also argued that claim 1(b) covers products with magnesium stearate because the ’720 patent examples disclose magnesium stearate in the outer matrix. The Court reiterated “the exceptionally strong presumption” that Markush groups are closed in rejecting the argument.
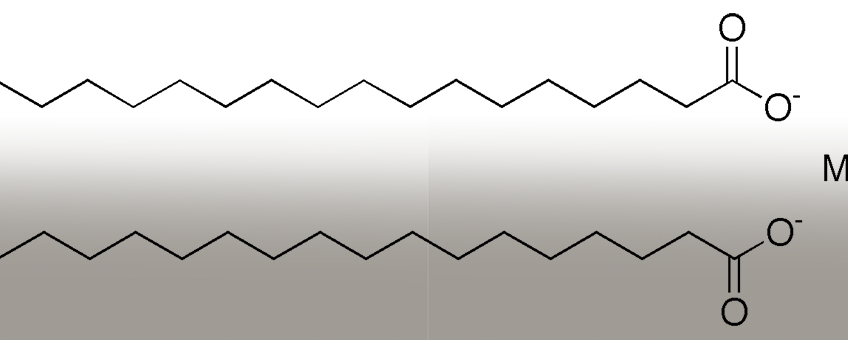
Images of chemical compound magnesium stearate from wikipedia.
Federal Bar member attorneys may access the full case summary by registered patent attorney B.C. “Bill” Killough in the March 2017 issue of Federal Circuit Case Digest.
 B.C. Killough is a registered patent attorney based in Charleston, SC. On behalf of his clients, Bill has obtained more than 300 United States patents, participated in prosecuting more than 100 foreign patent applications and he has filed more than 1000 trademark applications with the US Patent and Trademark Offices.
B.C. Killough is a registered patent attorney based in Charleston, SC. On behalf of his clients, Bill has obtained more than 300 United States patents, participated in prosecuting more than 100 foreign patent applications and he has filed more than 1000 trademark applications with the US Patent and Trademark Offices.